Rate of Chemical Reaction
Rate of Chemical Reaction: Overview
This Topic covers sub-topics such as Rate of a Chemical Reaction, Threshold Energy, Negative Catalysis, Positive Catalysis, Slow and Fast Reactions, Effect of Temperature on Rate of a Reaction and, Effect of Concentration of Reactants on Rate of a Reaction
Important Questions on Rate of Chemical Reaction
The ratio of disappearance of B is The rate of reaction and rate of change in concentration of A and C would be:
The quantitative estimation of change in the rate of reaction with change in concentration does not always follow stoichiometric equation. Justify.
If for the formation of from and is , then for decomposition of mole of is _____.
Enter your correct answe as A, B or C.
In the reaction , if is increased by three times then the difference in the rate is _____ of the initial rate.
The above reaction was studied at by monitoring the concentration of in which initial concentration was and after half an hour became . The rate of production of is _______
(Nearest integer)
If the following reaction , rate constant is. If we start with then Conc. of after ten minutes is
At high temperatures, ethyl chloride produces and ethylene by the following first order reaction:
In an experiment, when the initial concentration of ethyl chloride was , the rate of the reaction was found to be m/s. What will be the rate of reaction if the initial concentration of ethyl chloride is ?
In the reaction : , the rate of disappearance of B is . Find the rate of appearance of C.
In the reaction : , the rate of disappearance of B is . Find the rate of reaction.
The rate of reaction for is when . The rate of reaction when , in the same unit is ;
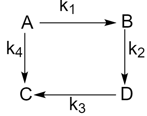
For an elementary reaction sequence shown, which of the following equation is correct?
The reaction, is carried out in a closed vessel. if partial pressure of is decreasing at the rate of . what is the rate of change of total pressue of vessel (in torr/min)
In the process rate of disappearance of A is & rate of appearance of B is at same instant. Then values of n & m respectively are
For the reaction, , it is observed that
The correct relation between is :
For a reaction , if the rate of consumption of A is , the rate of formation of Z will be
Consider the reaction :
If concentration of decreases from to in second, the rate of formation of is in the above reaction would be (In ).
Which of the following statement is correct for a reaction Products
For the reaction , write the rate of reaction expression.
Rate of reaction is the change in concentration of any one of the reactants or any one of the products in unit time. Express the rate of the following reaction in terms of reactants and products:
Write any four differences between 'rate of reaction' and 'rate constant' of a reaction.
